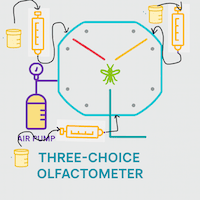
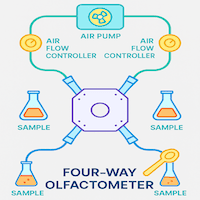
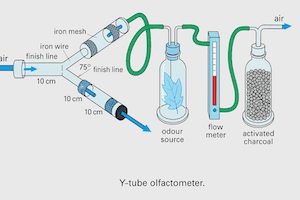
Clip Cages for Contained Studies on Insects
Clip cages, also known as insect cages or bug boxes, are small enclosures used in entomology to safely contain and study live insects. These cages are typically made of clear plastic or mesh material and feature a hinged lid or a sliding door for easy access. Clip cages provide entomologists and insect enthusiasts with a controlled environment to observe, study, and transport insects without the risk of escape or damage. Clip cages, also known as plant cages or growth cages, are useful tools in plant research for a variety of purposes. Here are some common uses of clip cages in plant research:
The uses of clip cages in entomology are diverse and valuable. Here are some common applications:
Observation and Study: Clip cages allow researchers and enthusiasts to closely observe insect behavior, feeding habits, mating rituals, and life cycle stages. By providing a contained environment, these cages offer a controlled setting for conducting experiments and making detailed observations.
Temporary Housing: Clip cages are useful for temporary housing of collected insects, such as those captured during fieldwork or sampling trips. They offer a secure and portable solution to transport insects back to the laboratory or study site without causing harm or disturbance.
Breeding and Rearing: Many entomologists use clip cages for breeding and rearing insects. By placing a mated female or a group of insects within a cage, researchers can monitor the mating process, egg-laying, and subsequent development of larvae or nymphs. This controlled environment allows for the study of population dynamics and provides an opportunity to collect valuable data.
Display and Education: Clip cages are commonly used in educational settings, museums, and insect exhibits to showcase live specimens. They offer a safe and interactive way for the public to observe and learn about various insects without direct contact. Clip cages can be designed to accommodate specific insect species or habitats, providing a visually appealing display.
Insect exclusion studies: Clip cages are often used to protect plants from insect herbivory or to exclude specific insects for controlled experiments. The cages prevent insects from accessing the plants while allowing air, light, and water to pass through. This enables researchers to study the impact of insect feeding or absence of insects on plant growth, physiology, and defense mechanisms.
Pollination studies: Clip cages can be used to control pollination in plants. By enclosing the flowers or inflorescences within the cages, researchers can prevent natural pollinators from accessing them. This allows for controlled hand-pollination experiments, where specific pollen sources can be applied to the flowers to study the effects on seed production, fruit development, or hybridization.
Seed collection: Clip cages can be employed to collect seeds from plants while preventing natural dispersal. By enclosing the seed heads or fruits within the cages, researchers can capture seeds as they mature and prevent them from being dispersed by wind or animals. This is particularly useful for plant breeding programs, seed production, or studies on seed dormancy and germination.
Herbicide or chemical applications: Clip cages can be used to study the effects of herbicides or other chemicals on plant growth and development. By enclosing the plants within the cages, researchers can ensure that the chemicals are applied specifically to the target plants while minimizing their contact with neighboring plants or the environment. This allows for precise dosage control and assessment of the effects of the chemicals on the target plants.
Physical manipulation experiments: Clip cages provide a controlled environment for physical manipulation experiments on plants. For example, researchers can use the cages to study the effects of bending, pruning, or other mechanical stresses on plant growth, branching patterns, or hormone distribution. The cages keep the manipulated plants separate from the surrounding vegetation, allowing for accurate measurements and observations.
When working with clip cages in entomology, it's important to follow some recommendations:
Size and Ventilation: Choose an appropriate size cage depending on the species and number of insects you are housing. Ensure adequate ventilation through small holes or mesh screens to maintain proper airflow and prevent overheating or suffocation.
Substrate and Food: Provide a suitable substrate or habitat within the cage that resembles the insect's natural environment. Depending on the species, this may include soil, leaves, branches, or specific plant species. Additionally, provide appropriate food sources to sustain the insects during their stay.
Cleaning and Maintenance: Regularly clean the clip cages to maintain hygiene and prevent the buildup of waste or harmful bacteria. Replace the substrate and food sources as needed to ensure the well-being of the insects.
Environmental Conditions: Consider the environmental conditions necessary for the particular insect species you are studying or housing. Temperature, humidity, and light exposure may vary depending on the insects' natural habitat, so try to replicate those conditions within the clip cage as closely as possible.
Clip cages play a vital role in the field of entomology, facilitating research, education, and conservation efforts. By providing a safe and controlled environment for live insects, these cages enable entomologists to gain valuable insights into the intricate world of insects while promoting their welfare and preservation.
Study of effects of biochemicals and pesticides on insects
To study the effects of insecticides on insects, researchers often use clip cages as a controlled environment to observe and analyze the impact of the chemicals. Clip cages are small enclosures made of mesh or fine netting that can be attached to plants or other substrates. They allow researchers to isolate and monitor individual insects while exposing them to specific treatments.
Here's a general procedure for using clip cages in studying insecticides on insects:
Select appropriate clip cages: Choose clip cages that are suitable for the size and type of insects you are studying. Ensure that the mesh or netting used in the cages is fine enough to prevent the insects from escaping.
Prepare the test area: Identify the area where you will conduct the study. It can be a field, a greenhouse, or a controlled laboratory setting. Make sure the area is free from other potential sources of contamination.
Set up the clip cages: Attach the clip cages to the desired plants or substrates using clips, ties, or any other suitable fasteners. Ensure that the cages are secure and cannot be easily dislodged.
Introduce the insects: Carefully introduce the insects you want to study into the clip cages. You may need to gently catch or collect them from the environment or obtain them from a reliable source.
Apply the insecticide: Follow the instructions and safety guidelines provided by the manufacturer for the specific insecticide you are using. Apply the insecticide to the plants or directly onto the insects within the clip cages. Ensure that you have a control group without any insecticide treatment for comparison.
Monitor the insects: Regularly observe and record the behavior, mortality, and other relevant parameters of the insects inside the clip cages. You can use visual observation or more advanced techniques like video recording or automated tracking systems.
Analyze the data:
Selection of parents for crop improvements especially against insect pests, and studying of economic threshold levels under controlled conditions (ETLs)
Using clip cages to screen germplasm for insect-resistant varieties is a common practice in agricultural research and breeding programs. Clip cages are small, enclosed structures made of mesh or fine netting that can be attached to plants to create a controlled environment for studying insect-plant interactions.
Here's a step-by-step process on how clip cages can be utilized to screen germplasm for insect resistance:
Selection of Germplasm: Identify the germplasm or plant material that you want to screen for insect resistance. This could include different varieties, hybrids, or genetically modified plants.
Insect Pest Selection: Choose the specific insect pest or pests that you want to evaluate the germplasm against. This could be a major pest that causes significant damage to the crop.
Clip Cages: Buy clip cages with fine netting material. The cages should be large enough to enclose individual plants or plant parts, such as leaves or stems. The mesh size should be small enough to prevent the chosen insect pests from entering or feeding on the enclosed plant material.
Attachment of Clip Cages: Attach the clip cages to the selected plants, ensuring that the cages are securely fastened. You may need to use clips, ties, or other methods to ensure a tight seal around the plant material.
Introduction of Insect Pests: Introduce the chosen insect pests into the clip cages. This can be done by manually transferring the pests or by placing eggs, larvae, or adults within the cage. Ensure that the insect population is representative of the pest pressure experienced in the field.
Monitoring and Evaluation: Regularly monitor the plants inside the clip cages for insect feeding, damage, or any signs of resistance. Record observations such as the number of pests, feeding behavior, plant damage, and overall plant health. Monitor the duration of the experiment according to the life cycle of the chosen insect pest.
Data Analysis: Analyze the data collected during the monitoring phase to assess the level of resistance or susceptibility in the different germplasm lines. Compare the performance of different varieties or hybrids based on the observed pest damage and plant health parameters.
Selection of Resistant Varieties: Based on the data analysis, identify germplasm lines that exhibit a higher degree of resistance to the target insect pest. These resistant varieties can be further evaluated and potentially used in breeding programs to develop insect-resistant cultivars.
By using clip cages, researchers can create a controlled environment to evaluate the performance of germplasm lines against specific insect pests. This allows for the identification and selection of insect-resistant varieties, which can contribute to the development of more resilient and productive.
Study of effects of insects on life table parameters of plant species
Clip cages, also known as exclusion cages, are commonly used in ecological studies to investigate the effects of different factors on plants and other organisms. These cages are typically constructed using a mesh material that allows air, light, and water to pass through while preventing access by specific organisms, such as insects or larger herbivores.
When studying the effects of herbivores, clip cages can be used to examine the impact of herbivory on plant growth, reproduction, and other ecological processes. By excluding herbivores from specific plants or plant populations, researchers can observe the differences in plant traits, such as leaf damage, flower production, or overall biomass, between caged and uncaged plants.
To study the ETLs (Herbivore-induced Effects on Plant Traits) of plants using clip cages, you can follow these general steps:
Select a study site and the plant species you want to investigate. Ensure that the chosen species has herbivores known to affect its traits.
Set up a replicated experimental design. Divide the study area into treatment plots, with some plots having clip cages and others without cages (control plots).
Construct the clip cages using appropriate mesh material (Buy from here). The mesh size should be small enough to exclude the target herbivores but large enough to allow for the passage of air, light, and precipitation.
Install the clip cages over the selected plants or plant populations in the treatment plots, ensuring that the cages completely enclose the plants.
Monitor the plants regularly, noting any changes in plant traits. Some common traits to measure or observe include leaf damage, biomass, reproductive output (flowering, fruit production), or specific chemical compounds (secondary metabolites) produced by plants in response to herbivory.
Repeat the measurements and observations over a suitable time period to capture the full extent of herbivory and its effects on plant traits.
Compare the plant traits between the caged and uncaged plants to assess the ETLs. Statistical analyses, such as t-tests or analysis of variance (ANOVA), can be employed to determine if there are significant differences between the treatments.
Consider additional factors that may influence the observed plant traits, such as abiotic factors (e.g., light availability, soil moisture), and control for these factors in your analyses if necessary.
Interpret the results and draw conclusions about the herbivore-induced effects on the plant traits of interest.
Remember to document your methods, including the specifics of the clip cages used and any other relevant information, to ensure the reproducibility of your study.